CMC
Using validated methods, Next Breath designs and conducts characterization studies
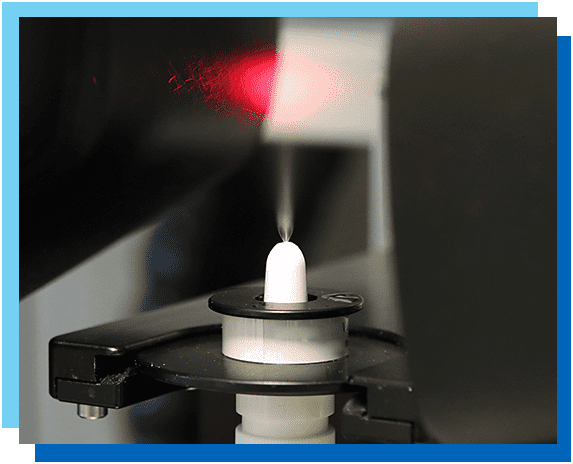
Next Breath’s CMC lab offers comprehensive Chemistry, Manufacturing and Controls testing services using the latest instrumentation and methodologies for metered-dose inhalers (MDIs), dry powder inhaler (DPIs), nebulizers, and nasal inhalation products. At the completion of each project, we provide our customers with detailed reports ready for regulatory submission.
Next Breath CMC Services:
- Actuation Force
- ICH Stability
- Clinical Batch Release
- Process Validation
- Temperature Cycling
- Prime Reprime
- Effect of Dosing Orientation
- Cleaning
- Device Robustness
- Photostability
- Priming and Repriming
- Device Profiling
Methods available with any service:
- Surface Tension
- Viscosity
- Moisture Content
- Osmolality
- Microbial limits
- Particulate Matter
- Identification
- Aerodynamic Particle Size
- Dose Content
- Uniformity
- Spray Pattern
- Plume Geometry
- Shot Weight
- Assay
- Impurities and Degradation
- Related Substances
- Preservative Assay
- Particle Size
- Droplet Size by Laser Diffraction
- Appearance
- Color
- Weight loss
- Net Content
- Nasal Cast Deposition
- Actuation Force
DISCOVER NEXT BREATH'S EXPERTISE
We’re here to help. Our experienced team of professionals are just a call or email away.
